Just to be clear about my own position. I have no reason to criticize Wizzzard’s recommendations, and I do respect that in the field of analytical chemistry his knowledge is much greater than mine. However, before he laid down his wisdom here, I have been using 25% IPA plus about 0.1% Triton X100 exclusively for more than two decades. Any LP I have ever washed was washed with the foregoing solution. So naturally when Wizzzard implies that IPA is potentially damaging, I want to know why and how and what would be the consequences. It is not enough, at least for me, just to be told that only Ethanol among all other alcohols and water do not damage vinyl. If Wizzzard does not have the information, that is OK by me, and he only needs to say so. But I object to being excoriated for asking.
The "Very Best Record Cleaning Formulation"
The "Very Best Record Cleaning Formulation"
I am providing this formulation for all who are interested in the very best, and can be proven and demonstrated to be the "Very Best". It can easily be made from available ingredients. On the surface, it appears to be very simple. However, it is based on extensive complex chemistry along with precise mathematical calculations and verifiable data.
You may use it with absolute confidence and be truly assured that it is beyond doubt the "Very Best". You may use it for your personal needs. Or, archival entities may use it for their purposes with confidence. Or, you may choose to start an enterprise that makes and packages quantities as either a "ready-to-use" or a "Semi-concentrated" version for sale and distribution knowing that nothing better exists. You have my blessings and encouragement with one condition. And, that is, that the pricing represents a "fair margin", and, not an obscene gouging, typical for such products.
Initially, I had prepared a presentation that briefly introduced myself, and provided the thought processes, design parameters, and the necessary basics of chemistry, physics, and mathematics to assure you and allow you to be absolutely confident in this formulation. I made a considerable effort to keep it as simple, but, also as thorough enough to achieve this confidence. However, that presentation entailed 5,239 words, typical of such a requirement, however, unacceptable in length by this website forum.
I have no option other than to offer the formulation as a 100% parts by weight version suitable to produce 1 Kilogram of the cleaner, and, invite you to question me about any aspect of the formulation.
Professionally, I am a Chemist, more specifically a Polyurethane Chemist. I have a Doctorate in Chemistry as well as two other Doctorates and a M.B.A.. I held prominent positions in significant corporations before being encouraged to start our (wife and I) manufacturing facility servicing those I previously worked for. We started, owned, and fully operated this business. We eventually obtained 85+% Market Share in our sector in Medical, Automotive, Sporting Goods, and Footwear areas before retirement.
The Audio Industry is extremely technical and many brilliant minds have contributed their talents over the decades in order that we may enjoy music today as we choose. Like many other technical industries, those of lesser minds and values invade the arena with their "magical" inspired revelations and offer their "magical" ingredients and items to all at extremely high prices. They promise that if only we are willing to part with our money - they can provide these items to you that make your audio system sound as if the orchestra, or vocalist, is in your room with you. And, after all, "magical items" must be expensive, otherwise, they would not be "magical".
This disturbs me enormously, and, it is for such reasons, I feel compelled to provide realistic and truthful information that conforms to basic Engineering, Chemistry, Physics, and Mathematical Principals in those areas with which I am very knowledgeable and familiar.
"Ultimate Record Cleaner Solution"
Ingredient Amount by Weight (Grams)
Distilled Water 779.962
Ethyl Alcohol 220.000
Tergitol 15-S-7 (Dow Chemical) 0.038 (Approx. = 2 Drops)
1,000.000
Important and/or Relevant Criteria
1.) Distilled Water ONLY. Do not use deionized, tap, rain, or spring water. Distilled Water is readily available in most grocery stores. Check labeling to be certain that it is distilled and not deionized. The pricing is comparable.
2.) Ethanol must be purchased at a "Liquor Store" or a "Liquor Control Board" that is suitable for human consumption, and the appropriate taxes must be paid. This assures that the alcohol consists of only Ethyl Alcohol and water. You need to purchase the 95+% version, also known as 180+ Proof. NOTHING ELSE is acceptable. (100% Ethyl Alcohol is not available under "normal" circumstances). Denatured alcohol from a Hardware Store or elsewhere is PROHIBITED, as well as ANY other alcohols.
3.) Tergitol 15-S-7 is made by Dow and is available on the internet in small quantities from Laboratory Supply Houses such as Fisher and Advance, etc.. I have no affiliations with either Dow Chemical, or Fisher, or Advance. You MUST use Tergitol 15-S-7 ONLY. No other Tergitol product is acceptable for this designed formula, and you need to acquire the undiluted form only.
4.) The above cleaner formula will result in a non-foaming (VLF) Surfactant Formulation that exhibits the following:
Surface Tension of 28.5 dynes/centimeter @ 20 C. (68.0 F.)
Surface Tension of 28.2 dynes/centimeter @ 25 C. (77.0 F.)
5.). A Surface Tension of 28.5 dynes/centimeter is Remarkable and will properly clean records of all organic soilings, and all oily substances, as well as very significant amounts of inorganic soilings. This available Surface Tension coupled with the Azeotropic Characteristics of very rapid evaporation and spotless drying occur because of the selection of Ethyl Alcohol and the very specific concentration determined as 22.00% p.b.w., further improves the products abilities. The "Ease-of-Use" and "Spot-Free" results are to be accepted.
6.). Be aware that an "ideal temperature of use" also exists for this formulation. And, that reasonable temperature is 40 C. (104.0 F.). Further increases in temperature offers no improvement, therefore, confirming the proper use of the term "ideal". I mention this not because of of any substantial improvement, but, only to be aware of its’ existence. And, if you have a choice to utilize a room that is warmer than another, select the warmer room closer to 104.0 F. There is no need to elevate the temperature of the records or the materials. Simply be aware that 104.0 F. Is ideal.
If interest is expressed in this submission, I am willing to provide additional submissions regarding other materials, and, other areas of interest. Such as"Best Contact Substance", "Best lubricants for turntables", " Better Dampening Materials" for turntables and tonearms, and, most significantly, "Best" material for "Turntable Platter/Vinyl Record Interface" usually called "Record Mats". The last item will certainly disturb many individuals and anger many suppliers.
Whatever I may contribute is substantiated by Science and Testing, and Verifiable. Science has no Opinions. Opinions in these matters are best reserved for those who rely on their imagination and wishful thinking.
Also, I have no vested interests in this Industry. Simply possess some scientific knowledge that also relates to some aspects of the Audio Area, and I am willing to share that information if requested!
- ...
- 470 posts total
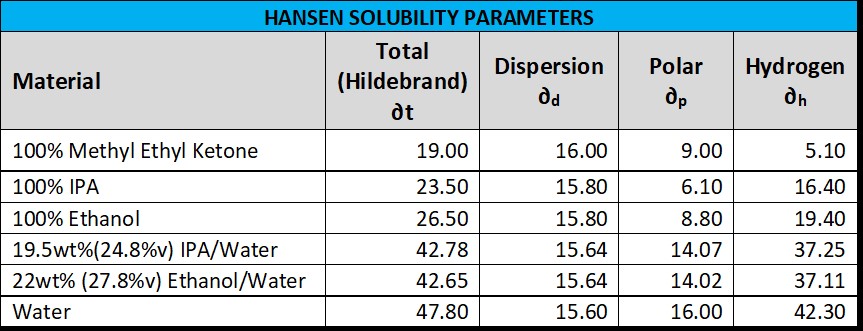
In polymer science there is an entire field of study surrounding the Hansen Solubility Parameters with a brief introduction here: Mechanical Properties of Polymer Nanocomposites (turi.org). There is a large handbook available to purchase just search Hansen Solubility Parameters Handbook. Below is a table showing the Hansen Solubility Parameters for various solvents. The % volume in parenthesize for the alcohol-water is just a specific gravity correction such as IPA is here - Density and Concentration Calculator for Mixtures of Isopropyl Alcohol and Water (handymath.com).
Without going into the details, if you were to model the record which is a co-polymer of PVC and PVA, determine its solubility sphere radius, and then compare with the alcohol-water solvents above using the Hansen procedures, you would see that the alcohol-water solvents are a safe distance away with no real risk of damaging the record (at room temp) consistent with many users experience. Note that when building the record model, it’s important to do a stepped proportional analysis where PVCa at the allowable variation using the RCA patent as a guide (1498409551006799538-03960790 (storage.googleapis.com) is first determined. Otherwise, doing just an analysis of the total PVC + PVA will yield a solubility sphere much larger making the record appear less compatible than it likely is based on years of user experience. Otherwise, how the record will be attacked by a solvent follows a fairly well-defined path - The paper A review of polymer dissolution, Beth A. Miller-Chou, Jack L. Koenig, Prog. Polym. Sci. 28 (2003) 1223–1270 states: “First, the solvent begins its aggression by pushing the swollen polymer substance into the solvent, and, as time progresses, a more dilute upper layer is pushed in the direction of the solvent stream. Further penetration of the solvent into the solid polymer increases the swollen surface layer until, at the end of the swelling time, a quasistationary state is reached where the transport of the macromolecules from the surface into the solution prevents a further increase of the layer.”. So, for a polymer, evidence of swell and maybe weight gain should be the first evidence of attack. What about extracting plasticizer - that should be unlikely. From the RCA Patent the small amount of plasticizer used is 1% of a soybean oil epoxide (ESO). Plasticizers can migrate from polymers based on three general mechanisms 1) evaporation to the ambient – same as off-gassing; 2) extracted by being soluble with liquids in contact; and 3) transfer from one surface of another. If the record had any significant % plasticizer it could never last as long as it does, and the ESO plasticizer is very stable. The paper Kinetics Study of the Migration of Bio-Based Plasticizers in Flexible PVC, Ching-Feng Mao and De-Bin Chan, 2012 International Conference on Life Science and Engineering IPCBEE vol.45 (2012) tested the migration of five different plasticizers (at concentrations about 30%) from very thin flexible PVC of 1 mm under contact with polystrene sheets at 190°C for 10 min. The plasticizers tested were acetyl tributyl citrate (ATBC), di (2-ethylhexyl) phthalate (DEHP), di (2-ethylhexyl), adipate (DEHA), and epoxidized soybean oil (ESO). The PVC/DEHP weight loss was about 2%, PVC/ATBC was about 7% weight loss, PVC/DEHA was about 12% weight loss, and the PVC/ESO showed no weight loss. Most of the above was excerpted from the book if that is of any interest. Regardless, above are sufficient references to read on your own, and hopefully guide you on you making your own assessment. Enjoy the deep-dive. |
- 470 posts total