In polymer science there is an entire field of study surrounding the Hansen Solubility Parameters with a brief introduction here: Mechanical Properties of Polymer Nanocomposites (turi.org). There is a large handbook available to purchase just search Hansen Solubility Parameters Handbook. Below is a table showing the Hansen Solubility Parameters for various solvents. The % volume in parenthesize for the alcohol-water is just a specific gravity correction such as IPA is here - Density and Concentration Calculator for Mixtures of Isopropyl Alcohol and Water (handymath.com).
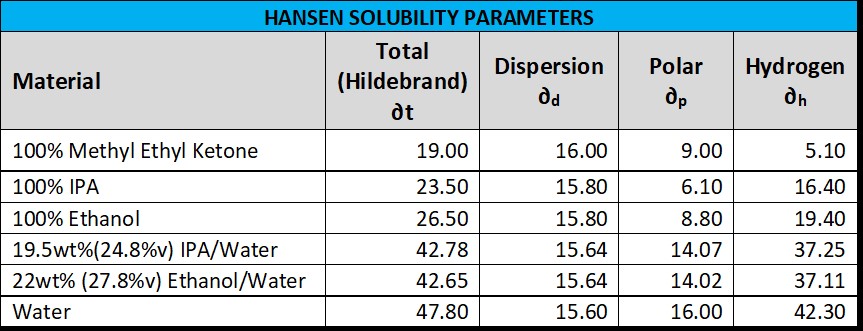
Without going into the details, if you were to model the record which is a co-polymer of PVC and PVA, determine its solubility sphere radius, and then compare with the alcohol-water solvents above using the Hansen procedures, you would see that the alcohol-water solvents are a safe distance away with no real risk of damaging the record (at room temp) consistent with many users experience.
Note that when building the record model, it’s important to do a stepped proportional analysis where PVCa at the allowable variation using the RCA patent as a guide (1498409551006799538-03960790 (storage.googleapis.com) is first determined. Otherwise, doing just an analysis of the total PVC + PVA will yield a solubility sphere much larger making the record appear less compatible than it likely is based on years of user experience.
Otherwise, how the record will be attacked by a solvent follows a fairly well-defined path - The paper A review of polymer dissolution, Beth A. Miller-Chou, Jack L. Koenig, Prog. Polym. Sci. 28 (2003) 1223–1270 states: “First, the solvent begins its aggression by pushing the swollen polymer substance into the solvent, and, as time progresses, a more dilute upper layer is pushed in the direction of the solvent stream. Further penetration of the solvent into the solid polymer increases the swollen surface layer until, at the end of the swelling time, a quasistationary state is reached where the transport of the macromolecules from the surface into the solution prevents a further increase of the layer.”. So, for a polymer, evidence of swell and maybe weight gain should be the first evidence of attack.
What about extracting plasticizer - that should be unlikely. From the RCA Patent the small amount of plasticizer used is 1% of a soybean oil epoxide (ESO). Plasticizers can migrate from polymers based on three general mechanisms 1) evaporation to the ambient – same as off-gassing; 2) extracted by being soluble with liquids in contact; and 3) transfer from one surface of another. If the record had any significant % plasticizer it could never last as long as it does, and the ESO plasticizer is very stable.
The paper Kinetics Study of the Migration of Bio-Based Plasticizers in Flexible PVC, Ching-Feng Mao and De-Bin Chan, 2012 International Conference on Life Science and Engineering IPCBEE vol.45 (2012) tested the migration of five different plasticizers (at concentrations about 30%) from very thin flexible PVC of 1 mm under contact with polystrene sheets at 190°C for 10 min. The plasticizers tested were acetyl tributyl citrate (ATBC), di (2-ethylhexyl) phthalate (DEHP), di (2-ethylhexyl), adipate (DEHA), and epoxidized soybean oil (ESO). The PVC/DEHP weight loss was about 2%, PVC/ATBC was about 7% weight loss, PVC/DEHA was about 12% weight loss, and the PVC/ESO showed no weight loss.
Most of the above was excerpted from the book if that is of any interest. Regardless, above are sufficient references to read on your own, and hopefully guide you on you making your own assessment. Enjoy the deep-dive.

